2017 Grand Prize and People’s Choice Award Winner – Jesse Adler
Designer Drugs
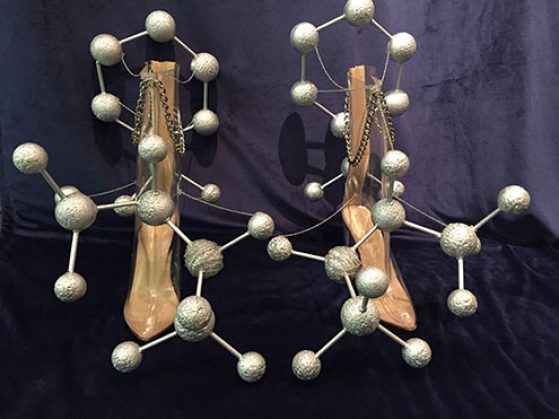
My true passion is the intersection between science and fashion, which is an emerging field that I aim to pioneer. For this reason, I chose to portray organic chemistry concepts in the form of shoes. Our bodies are mostly symmetrical, from head to toe, with one side mirroring the other. Although things like our hands and feet are perfect mirror images, they cannot be superimposed one upon the other. This principle is called chirality, and it can be used to describe molecules as well. It was my goal to use the chirality of our feet as a reflection of a molecule and its mirror image. The mirror image of a chiral molecule is called its enantiomer.
While our two feet have the same function practically, enantiomers do not. Since our bodies have very specified receptors, even a minute change in a molecule’s structure results in a different function or product. The molecule on the left shoe is dextromethamphetamine, more commonly referred to as “meth.” The right shoe displays the enantiomer of meth, called levomethamphetamine, which is the active ingredient in some over-the-counter nasal decongestants. While these two molecules are structurally identical, their exact opposite orientations result in major differences in the body. This is the reason that one does not become addicted to or experience euphoria from drugs such as Vicks VapoInhaler ™, and also why smoking meth will not clear up your congestion.
In 3-D space, a molecule is not always planar, thus it would have a specific orientation in space. At the same time, molecules rotate freely, meaning that sometimes a substituent can be viewed as if it is coming out towards you, but then it could rotate in a way that that same substituent is now going away from you. A way of attempting to conceptualize this 3-D concept in 2-D is a Newman Projection. This method views a carbon-carbon bond from front to back, as if you were staring down that bond, and therefore cannot see the bond itself. In Designer Drugs, the chosen carbon-carbon bond is the shoe itself. When you stare straight down the front of the shoe, at the carbon that is directly attached, you can see the Newman Projection. This technique allows chemists to visualize the possibilities of the molecule’s most stable conformation and also the direction in which it will rotate polarized light.
Every part of the construction of this piece was deliberate. For example, nitrogen and carbon atoms have similar atomic radii, as reflected in the sizes of the Styrofoam spheres. Yet, the nitrogen atom has chain wrapped around to differentiate it, and show that its atomic mass is greater than carbon. And thus, with the concepts of chirality, enantiomers and Newman Projections, I present Designer Drugs- an attempt to represent chemistry in a way that could be a part of an avant-garde runway show.
