2023 Honorable Mention – Jennifer Guo
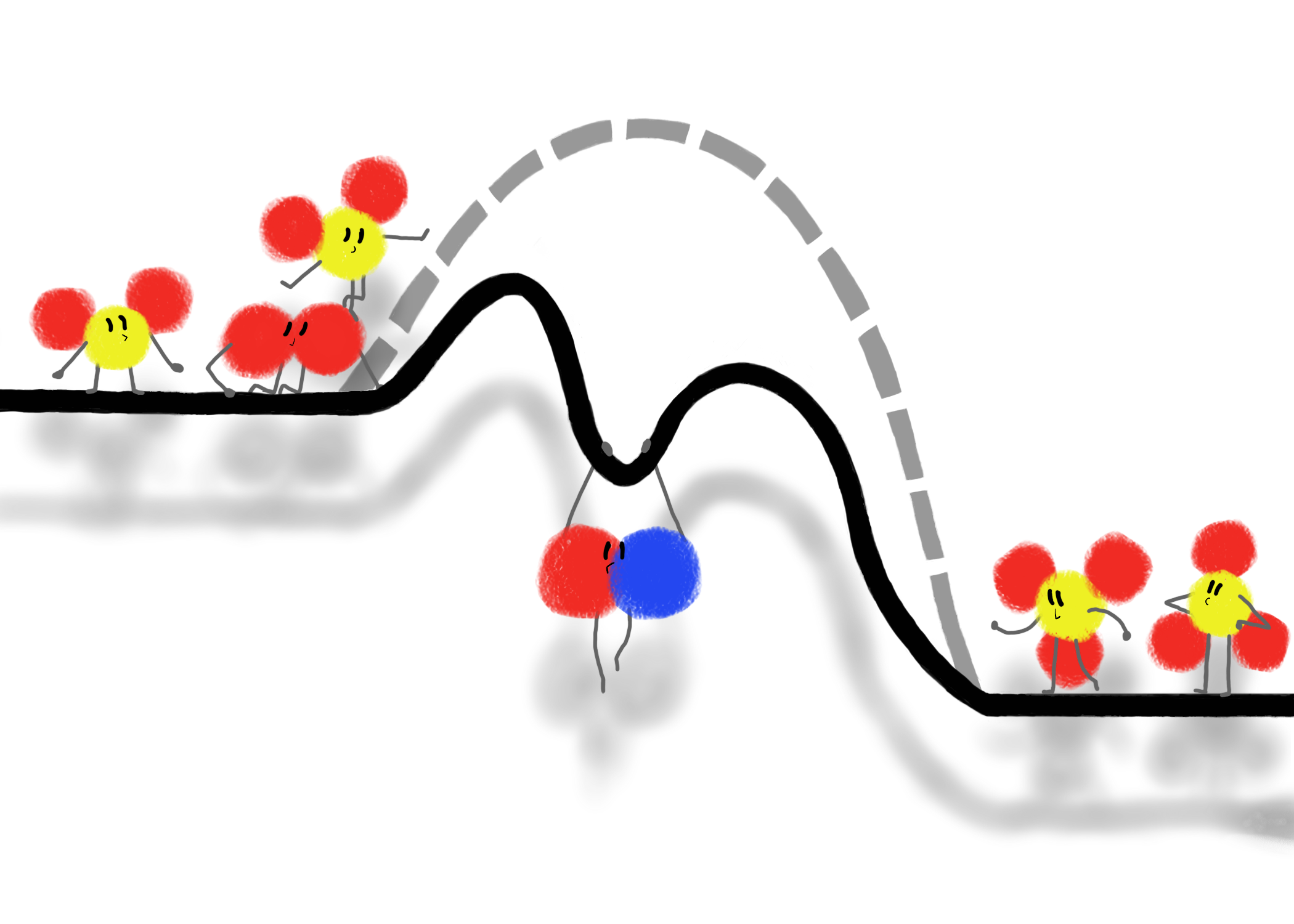
Move the Mountain
Digital illustration
Nitric oxide (NO) acts as a catalyst for the reaction 2 SO2 + O2 → 2 SO3. Catalysts are not able to bring the starting and ending energies of a reaction closer together. What they are able to do is provide a different pathway for the reaction to take, with lower energy barriers for the molecules to overcome. This allows the reaction to proceed at a faster rate. In a playful twist, a molecule of NO literally pulls down the curve of a potential energy diagram, as the reactants and products look on.
Catalysts are substances that are not consumed in a chemical reaction, but “speed up” the reaction rate. When a catalyst is present, the reaction progresses along a different set of steps, where the catalyst is often converted into an intermediate, but then converted back to its initial form in a later step, and therefore isn’t consumed in the full reaction. The example used in this piece is the reaction 2 SO2 + O2 → 2 SO3, which can be catalyzed by NO. The first step of the catalyzed reaction is 2 NO + O2 → 2 NO2, where NO is converted into NO2. The second step is NO2 + SO2 → NO + SO3. After the second step happens twice, both SO3 molecules from the overall reaction are produced and both original NO molecules are reformed.
When first teaching about catalysts in middle or high school, potential energy diagrams often show the lowering of a reaction’s activation energy, with a taller “peak” becoming a shorter one, but this isn’t entirely accurate. A more precise potential energy diagram has multiple “peaks”, one for each stage of the reaction. The activation energy of the new reaction (the one with the catalyst) is determined by the tallest peak, and is lower than the peak of the original reaction without the catalyst. When I learned about catalysts, I imagined the catalysts literally pulling down the activation energy, making it easier for the reactants to get over that “hump” and for the reaction to proceed. The intermediate dip between the two peaks felt like the right place for a catalyst to grab onto, pulling the curve down with their molecular weight.
As a kid, I personified all sorts of objects, concepts, and organizations. Countries, corporations, vehicles, buildings, and, once I started learning chemistry, molecules and elements of the periodic table. Making inanimate things into characters with wants and feelings helped me build stories around what I was learning, such as the one with the catalysts and the peaks, and thus absorb and remember the information better. My motivation as an artist is to show people what I see, in the hopes that it makes the world more interesting and memorable for others as well.
